- Shares are soaring for a newly-listed Nasdaq, GT Biopharma (NASDAQ:GTBPNASDAQ:GTBP), with a brand-new approach to treating cancer.
- Channeling immune response, this innovative treatment supercharges the body’s NK (Natural Killer) cells to slow the growth of deadly tumors. Phase 1 clinical trials have shown reductions in cancer cells as high as 63%.1
- What’s more, it’s a fraction of what similar cancer-fighting immunotherapies cost. And the best part is, this brand-new immunotherapy has virtually no side effects.
- Investors are starting to catch on. The company has seen a rapid 600% rise in share value over the last twelve months, peaking at $18 in June, before settling back to a healthy 200%+ gain.
Cancer remains a devastating scourge that affects approximately 39.5% of the global population at some point in their lifetime2.
According to the National Cancer Institute, approximately 18 million people are diagnosed with cancer – and 9.5 million die from the disease each year3.
Despite this, the main therapies used to treat cancer, are now more than 80 years old, with chemotherapy first becoming widely used in the 1940s,4 and radiation dating as far back as 18955.
Yet that appears to be changing rapidly, as a new class of immuno-oncology companies look to the body’s own natural defense system, Natural Killer (NK) cells, to fight one of the world’s most prolific diseases.
A Burgeoning Immuno-Oncology Industry
As a result, the market for immuno-oncology drugs and therapies is expected to grow from $62.2 billion in 2020 to $194 billion by 2028, growing at a rate of 15% per year.6
The largest share of the global market is North America, with much of the focus (72%) on treating cancer with monoclonal antibodies that attach themselves to lymphocytes such as NK cells.7
According to a Research and Markets report, there are around 100 companies and 140 new drug therapies in the Natural Killer Cell pipeline landscape.8 Many of which are already seeing staggering growth, as the sector quickly gains widespread attention.
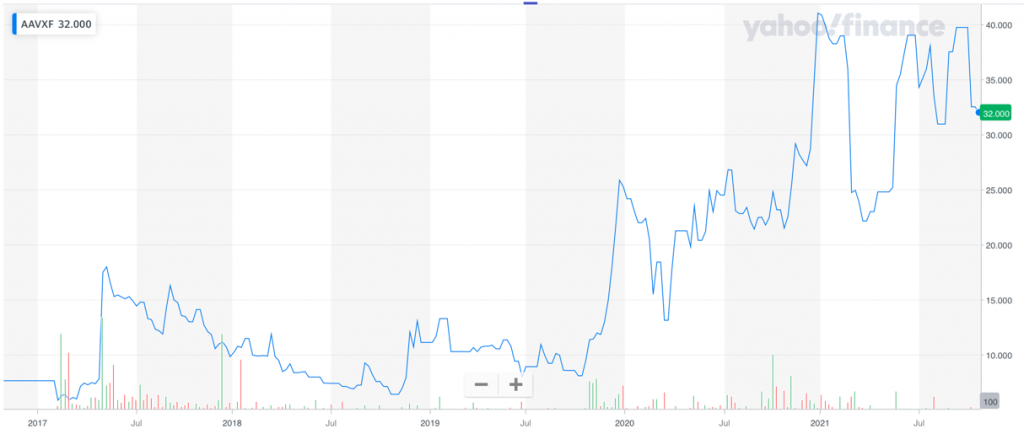
Shares of ABIVAX Société Anonyme (AAVXF), a French company that is currently testing an immune enhancer candidate now in clinical trials for the treatment of hepatocellular cancer, have risen 272%9 in the past two years, from $8.6010 a share in September 2019 to $32.11
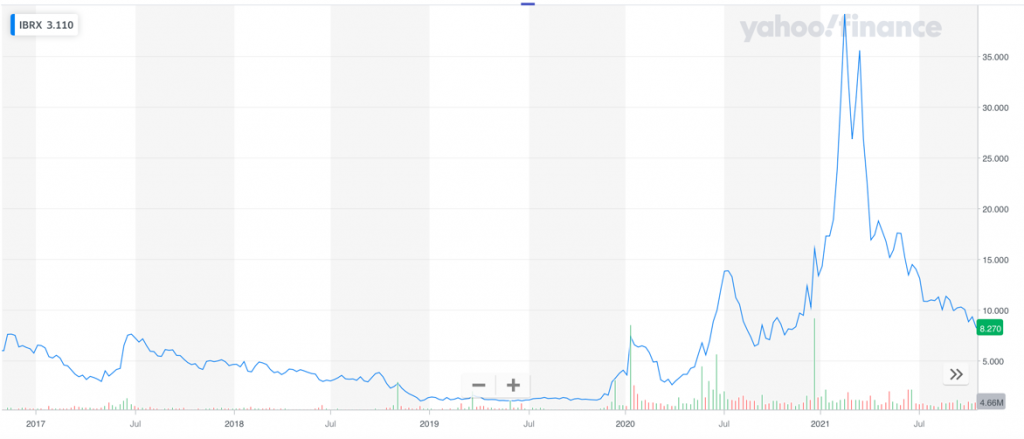
ImmunityBio, Inc. (NASDAQ: IBRX), a clinical-stage immunotherapy company with a new treatment for advanced metastatic pancreatic cancer, saw its shares jump from $1.0512 in October 2019 to as high as $1913 a share in April 2021 – a gain of 1,709%14 in just two years.
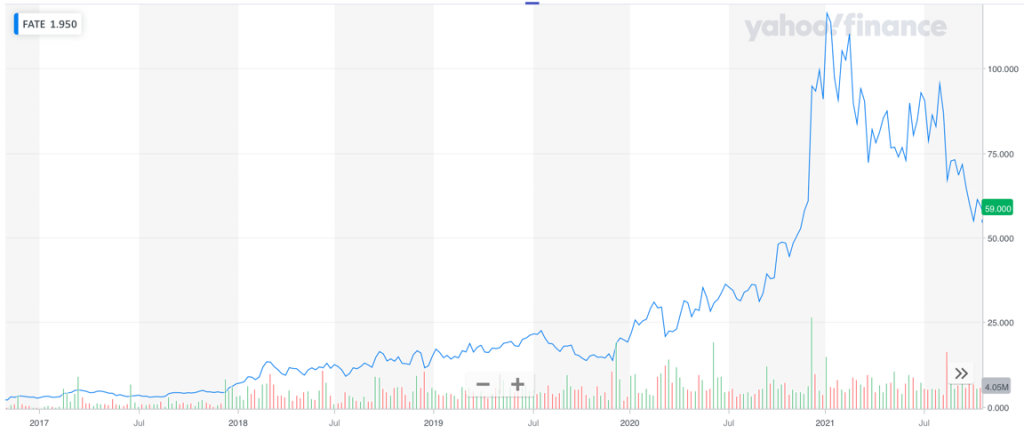
And Fate Therapeutics, Inc. (Nasdaq: FATE), a California company with NK- and T-cell immuno-oncology programs now being tested, has risen from $1515 a share in November 2019 to $6016 a share in October 2021 – another gain of 300%17 in under two years. Fate Therapeutics is currently developing a cell therapy that involves production of NK cells outside the body.
Unfortunately, these stocks have already seen huge gains – and are now selling at a premium.
That’s why investors are taking a close look at companies just coming online with newer, more effective treatments. And one particular upstart is just starting to gain widespread attention: GT Biopharma (NASDAQ:GTBPNASDAQ:GTBP).

GT Biopharma is an attractive target because it’s a small company with transformative potential, and its still flying under the radar of Wall Street.
Investors can load up for less than $10 per share. If the company’s stock takes off, those who get in early could potentially see enormous profits.
And GT Biopharma’s immunotherapy treatment is state-of-the-art, designed by the same scientist behind Fate Therapeutics’ immunotherapy, but utilizes a radically different method.
Unleashing the Body’s SEAL Team Defenses to Fight Against Cancer
What sets newcomer GT Biopharma (NASDAQ:GTBPNASDAQ:GTBP) apart is its proprietary protein technology, known as Trispecific Killer Engagers, trademarked TriKE®.
Originally developed by renowned immunologist, Dr. Jeffrey Miller of the University of Minnesota Medical School, TriKE® works by activating a special type of white blood cell, helping it identify and eliminate deadly cancer cells in the body.
The TriKE proteins, delivered to a patient intravenously18, are designed to trigger a “marker” on a patient’s tumor cells and then tell “natural killer” (NK) cells in the patient’s body to attack the marked cells.
Unlike some conventional chemotherapy treatments, which often attack both healthy cells along with cancer cells, GT Biopharma’s TriKE agents are designed for precision, programming NK cells to attack only tumor cells and to leave healthy cells intact.
This helps to reduce the body’s own, often debilitating immune system response that happens when cancer cells are attacked.
There are two main types of cancer-fighting lymphocyte (white blood) cells in a human body: T-cells and NK (Natural Killer) cells.
The T-Cells are like ordinary infantry soldiers. They can attack tumor cells and may or may not win any given battle. The problem with T-cells is that, if you get too many of them fighting all at once, they can cause massive destruction in their own right – what doctors call a “cytokine storm” that can also kill patients.
The NK cells are different. They are like elite commandos carrying vast quantities of explosives that can destroy whatever they target.
Most biotech companies working in the field are trying to harness the power of the NK cells to fight cancer. The problem is, there aren’t as many NK cells in the body as T-cells – and once their primary target is destroyed, they’re exhausted and cease fighting.
As a result, many biotech companies developing immunotherapy treatments for cancer try to create new NK cells in a lab. But this is a time-consuming process that is hugely expensive and often very hard on patients.
Supercharging Existing NK Cells
Based on Dr. Miller’s latest breakthrough, GT Biopharma (NASDAQ: GTBP), is working towards a radically new approach. Its proprietary protein technology does four very important things:
- First, the TriKE® protein activates or awakens the body’s own NK cells.
- Second, it helps the NK cells precisely target tumor cells and only tumor cells.
- Third, it allows the NK cells to continue attacking tumor cells, one after the other, for months instead of days.
- And fourth, it helps the NK cells proliferate or reproduce.
Putting the Body’s Defenders on Steroids
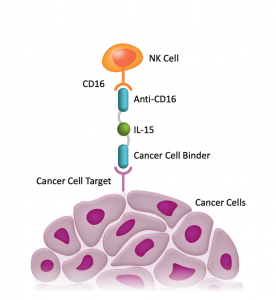 GT Biopharma’s “secret sauce” is the unique way it is able to embed a compound known as interleukin (IL-15) within its TriKE® protein at 17 times19 the normal dose and yet without side effects.
GT Biopharma’s “secret sauce” is the unique way it is able to embed a compound known as interleukin (IL-15) within its TriKE® protein at 17 times19 the normal dose and yet without side effects.
This potent blast of interleukin acts like a kind of cellular steroid, greatly prolonging the cancer-destroying ability of the NK cells and helping them to proliferate.
The bottom line is that this is potentially a revolutionary breakthrough in the treatment of some types of cancer – and, if it works, the size of the market is enormous.
Data from early clinical trials are encouraging.
Stage 4 cancer patients saw virtually no side effects yet experienced significant reductions in the amount of cancer cells in their bodies.
Plus, the cost is low: Just $30,000 per patient (covered by many insurance policies) instead of the $2 to $3 million per patient that other NK cell immunotherapies can charge.
For investors, there’s something else: The TriKE® protein is a genuinely new “first in class” approach that makes GT Biopharma stand out from its competitors, even those with market caps in the billions.
Unlike companies such as Fate Therpeutics and Nkarta Therapeutics, BT Biopharma’s approach is not a cellular therapy but a simple drug. That means patients don’t have to pay for, and endure, highly customized lab-created treatments.
What’s more, GT Biopharma (NASDAQ:GTBPNASDAQ:GTBP)’s immunotherapy has virtually no side effects – and that, too sets it apart. For patients suffering from end-stage cancer, the ability to tolerate treatments is an important factor.
In addition, the TriKE® protein appears to naturally increase the number of NK cells within patient’s own bodies, potentially prolonging life.
A Small Company with a Breakthrough Technology
Most important for investors, the market cap of other NK cell immunotherapy companies are in the billions – for example, Fate Therapeutics, which markets a therapy based on Dr. Miller’s earlier research, has a market cap of $8.48 billion.
In contrast, GT Biopharma (NASDAQ:GTBPNASDAQ:GTBP) is a smaller company that has licensed and is developing Dr. Miller’s latest, most advanced immunotherapy – and so its market cap is only $300 million and growing fast.
That means early investors can acquire significant share positions at very low costs.
The company currently has not one but five publicly-disclosed TriKE products in the pipeline.
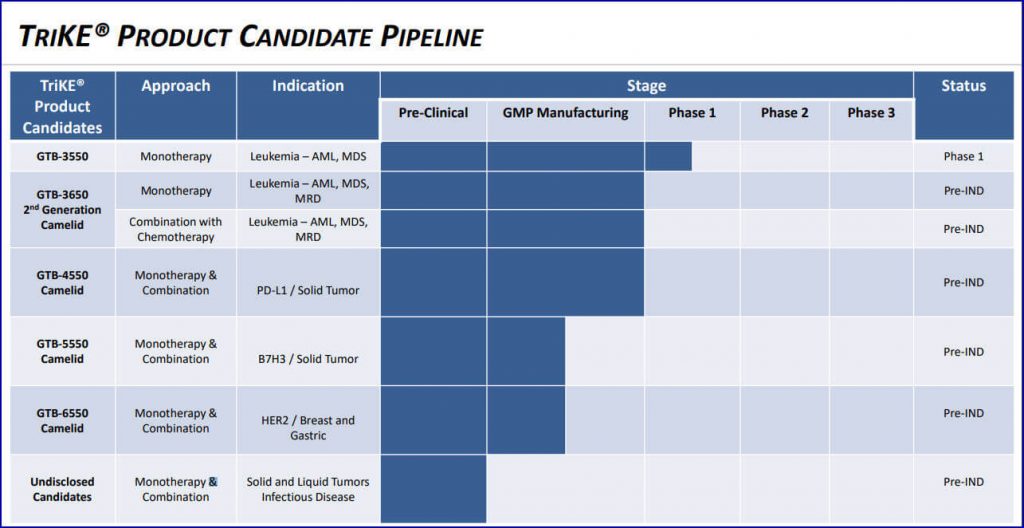
One, GTB-3550, the Company’s first TriKE product being evaluated for the treatment of leukemias such as acute myeloid leukemia (AML), myelodysplastic syndrome (MDS), and other CD33+ hematopoietic malignancies, has completed Phase 1 clinical trials and will soon begin Phase 2a.
Another, GTB-3650, is a second-generation protein biologic for treating AML and MDS, has completed pre-clinical testing and is now in the GMP manufacturing phase.
Two other products, GTB-4550 TriKE and GTB-5550 TriKE, designed to treat PD-L1+ and B7H3+ solid tumors respectively, will also begin clinical trials shortly. Plus, GTB-6550, for the treatment of gastric and breast cancers, is currently finished with pre-clinical testing and is now in GMP manufacturing.20
In addition, the company recently announced that it had won two U.S. patents for its products, specifically a patent for broad coverage for TriKE proteins targeting any antigen and another for broad coverage for TriKE proteins targeting HIV antigens.22 The company plans to advance these TriKEs into the clinic in 2022.
In other words, the time to act is right now.
If clinical trials of GT Biopharma’s TriKE® therapies show further promise, then all bets are off. Investors could potentially see substantial returns.
Of course, it goes without saying that there are no guarantees in biotech investing. Past performance is no guarantee of future results, and you should only speculate with money you can afford to lose.
Yet for investors who like stocks with a substantial risk/ reward ratio, then GT Biopharma, currently selling for less than $10 per share, may well be worth a close look.
For more information about GT Biopharma (NASDAQ: GTBP), visit the company’s website:
1 Patient #7, reducing MDS level from 12% to 4.5%, Video Presentation, minute 20:00 ff, https://vimeo.com/592449350
2 https://www.cancer.gov/about-cancer/understanding/statistics
3 https://www.cancer.gov/about-cancer/understanding/statistics
4 https://www.yalecancercenter.org/Answers_July_6_08_89594_5_v1.pdf
5 https://cancerres.aacrjournals.org/content/69/2/383
6 https://www.zionmarketresearch.com/report/immuno-oncology-therapy-market
7 Ibid.
8 https://ca.finance.yahoo.com/news/global-natural-killer-cell-therapies-143400862.html
9 https://www.calculator.net/percent-calculator.html?c3par1=8.60&c3par2=32&ctype=3&x=83&y=18#pctdifference
10 https://finance.yahoo.com/quote/AAVXF/history?
11 https://finance.yahoo.com/quote/AAVXF/history?
12 Closing price of $1.05 on October 31, 2019, see https://finance.yahoo.com/quote/IBRX/history?p=IBRX
13 Closing price of $19.33 on April 28, 2021, see https://finance.yahoo.com/quote/IBRX/history?p=IBRX
14 https://www.calculator.net/percent-calculator.html?c3par1=1.05&c3par2=19&ctype=3&x=49&y=26#pctdifference
15 https://finance.yahoo.com/quote/FATE/history?p=FATE
16 https://finance.yahoo.com/quote/FATE/history?p=FATE
17 https://www.calculator.net/percent-calculator.html
18 Verify
19 Webinar video, Minute 12:00 ff.
20 https://d1io3yog0oux5.cloudfront.net/_8422eae4a2802c98c45379aa35dd9978/gtbiopharma/db/184/625/pdf/GT+Biopharma+Corporate+Presentation+September+09-20-2021.pdf
21 https://www.gtbiopharma.com/news-media/press-releases/detail/234/gt-biopharma-announces-issuance-of-two-new-patents-covering
IMPORTANT NOTICE AND DISCLAIMER
This website is owned and hosted by Market Tactic Media Ltd. Articles appearing on this website should be considered paid advertisements. Market Tactic Media Ltd. and its owners, managers, employees, and assigns (collectively “the Website Host”) is often paid by marketing companies to host websites on which articles profiling public companies are published. The Website Host has not been compensated by any of the profiled companies. The Website Host’s compensation for articles appearing on this website is as follows:
- The Website Host has been paid approximately $500 per week while the advertisement campaign is active by Think Ink Marketing as compensation to host the article profiling GT Biopharma.
SHARE OWNERSHIP
The Website Host does not own any shares of any profiled GT Biopharma and has no information concerning share ownership by others of any profiled GT Biopharma. The Website Host cautions readers to beware that third parties, profiled companies, and/or their affiliates may liquidate shares of the profiled companies at any time, including at or near the time you read the articles on this website and this has the potential to hurt share prices. Frequently companies profiled in such articles experience a large increase in volume and share price during the course of investor awareness marketing, which often ends as soon as the investor awareness marketing ceases.
NO SECURITIES OFFERED
The articles on this website are not, and should not be construed to be, offers to sell or solicitations of an offer to buy any security. Neither the articles on this website nor the Website Host purport to provide a complete analysis of any GT Biopharma or its financial position. The Website Host is not, and does not purport to be, a broker-dealer or registered investment adviser. The articles on this website are not, and should not be construed to be, personalized investment advice directed to or appropriate for any particular investor. Any investment should be made only after consulting a professional investment advisor and only after reviewing the financial statements and other pertinent corporate information about the GT Biopharma. Further, readers are advised to read and carefully consider the Risk Factors identified and discussed in the profiled GT Biopharma’s SEC and/or other government filings. Investing in securities, particularly microcap securities, is speculative and carries a high degree of risk.
INDEMNIFICATION/RELEASE OF LIABILITY
By reading articles on this website, you acknowledge that you have read and understood this disclaimer, and further that to the greatest extent permitted under law, you release the Website Host, its affiliates, assigns and successors from any and all liability, damages, and injury from articles appearing on this website. You further warrant that you are solely responsible for any financial outcome that may come from your investment decisions.
LINKS TO THIRD PARTY WEBSITES
This website enables users to link to external websites not under the control of The Website Host. The Website Host has no control over the nature, content, and availability of those sites. The inclusion of any links is not intended as, and should not be construed as, a recommendation or endorsement of the content or views expressed on such external websites. The Website Host expressly disclaims any representation concerning the quality, safety, suitability, or reliability of any external websites and the content and materials contained in them. It is important for users to take necessary precautions, especially to ensure appropriate safety.
INTELLECTUAL PROPERTY
The Market Tactic is the Website Host’s trademark. All other trademarks used in this communication are the property of their respective trademark holders. The Website Host is not affiliated, connected, or associated with, and is not sponsored, approved, or originated by, the trademark holders unless otherwise stated. No claim is made by the Website Host to any rights in any third-party trademarks.
FORWARD LOOKING INFORMATION
This document contains forward-looking information and forward-looking statements, within the meaning of applicable Canadian securities legislation, (collectively, “forward-looking statements”), which reflect expectations regarding GT Biopharma future growth, future business plans and opportunities, expected activities, and other statements about future events, results or performance. Wherever possible, words such as “predicts”, “projects”, “targets”, “plans”, “expects”, “does not expect”, “budget”, “scheduled”, “estimates”, “forecasts”, “anticipate” or “does not anticipate”, “believe”, “intend” and similar expressions or statements that certain actions, events or results “may”, “could”, “would”, “might” or “will” be taken, occur or be achieved, or the negative or grammatical variation thereof or other variations thereof, or comparable terminology have been used to identify forward-looking statements. These forward-looking statements include, among other things, statements relating to: (a) revenue generating potential with respect to GT Biopharma industry; (b) market opportunity; (c) GT Biopharma business plans and strategies; (d) services that GT Biopharma intends to offer; (e) GT Biopharma milestone projections and targets; (f) GT Biopharma expectations regarding receipt of approval for regulatory applications; (g) GT Biopharma intentions to expand into other jurisdictions including the timeline expectations relating to those expansion plans; and (h) GT Biopharma expectations with regarding its ability to deliver shareholder value. Forward-looking statements are not a guarantee of future performance and are based upon a number of estimates and assumptions of management in light of management’s experience and perception of trends, current conditions and expected developments, as well as other factors that management believes to be relevant and reasonable in the circumstances, as of the date of this document including, without limitation, assumptions about: (a) the ability to raise any necessary additional capital on reasonable terms to execute GT Biopharma business plan; (b) that general business and economic conditions will not change in a material adverse manner; (c) GT Biopharma ability to procure equipment and operating supplies in sufficient quantities and on a timely basis; (d) GT Biopharma ability to enter into contractual arrangements; (e) the accuracy of budgeted costs and expenditures; (f) GT Biopharma ability to attract and retain skilled personnel; (g) political and regulatory stability; (h) the receipt of governmental, regulatory and third-party approvals, licenses and permits on favorable terms; (i) changes in applicable legislation; (j) stability in financial and capital markets; and (k) expectations regarding the level of disruption as a result of COVID-19. Such forward-looking information involves a variety of known and unknown risks, uncertainties and other factors which may cause the actual plans, intentions, activities, results, performance or achievements of GT Biopharma to be materially different from any future plans, intentions, activities, results, performance or achievements expressed or implied by such forward-looking statements. Such risks include, without limitation: (a) GT Biopharma operations could be adversely affected by possible future government legislation, policies and controls or by changes in applicable laws and regulations; (b) public health crises such as the COVID-19 pandemic may adversely impact GT Biopharma business; (c) the volatility of global capital markets; (d) political instability and changes to the regulations governing GT Biopharma business operations (e) GT Biopharma may be unable to implement its growth strategy; and (f) increased competition. Except as required by law, the Website Host undertakes no obligation to update or revise any forward-looking statements, whether as a result of new information, future event or otherwise.
HISTORICAL INFORMATION
Any graphs, tables or other information demonstrating the historical performance or current or historical attributes of GT Biopharma or any other entity contained in this document are intended only to illustrate historical performance or current or historical attributes of GT Biopharma or such entities and are not necessarily indicative of future performance of GT Biopharma or such entities.