This is long-awaited hope that could change the lives for hundreds of millions worldwide!
And the bridge to it all is a remarkable new diagnostic technology developed by Cognetivity Neurosciences (CSE: CGN | OTCQB: CGNSFCSE: CGN | OTCQB: CGNSF)
Now trading at under 60¢ Cognetivity holds stunning growth potential as its technology plays a key role in early diagnosis and ongoing management of new treatment protocols for Alzheimer’s patients!
Pharmaceutical giant, Biogen (NASDAQ: BIIB $41B), is now phase three trails for a drug that promises to stop the onset and progress of Alzheimer’s disease and related dementias.
On parallel tracks, Eli Lilly (NYSE: LLY $197B) is advancing a similar drug now in phase two trials and Cassava Sciences (Nasdaq: SAVA $2.12B) has entered phase three trials for its own Alzheimer’s drug, simufilam.




These three pharmaceutical giants are moving quickly toward the first ever medications to treat Alzheimer’s, a devastating disease that impacts hundreds of millions of people worldwide.
These drugs represent major breakthroughs, true miracles of medicine in the treatment of dementia illnesses. But their potentials are seriously impaired by antiquated diagnostic procedures that are over 100 years old.
The acceptance of new diagnostic and disease management protocols for Alzheimer’s is long overdue; that’s exactly what Cognetivity Neurosciences (CSE: CGN | OTCQB: CGNSFCSE: CGN | OTCQB: CGNSF) has developed.
Cognetivity’s newly released and regulatory approved Integrated Cognitive Assessment (ICA) technology is a breakthrough diagnostic tool necessary for early detection of Mild Cognitive Impairment (MCI) and the ongoing assessment of Alzheimer’s and other dementia-related illness.
In addition to early detection, Cognetivity’s ICA technology enables doctors to monitor a patient’s condition and adjust treatments in person and remotely for optimal outcomes.
Nothing like ICA can be found anywhere in medicine today and its introduction couldn’t be more timely and urgent.
Early diagnosis is the essential first step for any new Alzheimer’s drug therapy as is disease management. But recent clinical work has shown that long-standing diagnostic protocols are “not an optimal measure for pre-dementia studies”.
That assessment was published by the peer-reviewed Journal of Alzheimer’s Disease. It’s science jargon declaring the old methodologies functionally worthless!
Eli Lilly, Cassava, and Biogen should be keenly aware of this, which is why wide deployment and adaption of ICA technology could prove essential for their successful drug rollout.
Investors should note that in anticipation of FDA approval for any of these drugs, Cognetivity reports it intends to play an essential role as the “standardized test for accessing changes in cognition”.
Coinciding with announcements of their trial progress, Eli Lilly and Biogen saw sharp investor response following news of progress through FDA trials.
Eli Lilly, for example, is up about a third since August last year. That’s an impressive climb for a $197 billion company!
Cassava shareholders were the big winners though. On announcement of phase three trials, Cassava shares soared roughly 2,000% in the same time frame!
Clearly, investors appear to be intently watching these three companies and the huge breakthrough potential of these drugs.

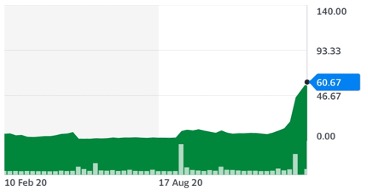
On news of its progress into stage three trials, Cassava Sciences shares rocketed over 1,940%, climbing from $3.12 in August last year to a stunning $60.67 high in mid-February!
What has not yet caught their attention though is the essential role that Cognetivity’s ICA technology holds ahead of these drugs entry to market.
Now is the time to get in front of this…the potential is simply staggering.
Think of it this way. You can’t prescribe a drug and manage treatment without effective diagnostic tools! The drug companies need these tools in doctors’ hands and that’s exactly where Cognetivity’s (CSE: CGN | OTCQB: CGNSF) ICA technology comes into play.
Cognetivity provides a 21st Century solution for managing modern medicine!
Cognetivity’s technology is already approved for market. It has passed critical approval stages for European markets and is poised for U.S. and worldwide distribution to over a half billion people worldwide using Apple iPad hardware platforms.
This is moving fast.
Cognetivity recently confirmed they were one of only seven health tech innovators hand picked to advance to TMCx full accelerator program. A rigorous multi-round selection process. 170 applicants from 20 different countries originally applied, with just seven standout healthcare innovators invited to join the full accelerator programme. The TMCx is dedicated to identifying breakthrough healthcare technologies and enabling their subsequent commercialization. Increased resources and network support will significantly accelerate Cognetivity’s US market penetration anticipated later this year.
 In the same timeframe, Biogen announced an alliance with Apple to study further development of early diagnostic tools using the Apple Watch and iPhone.[1]
In the same timeframe, Biogen announced an alliance with Apple to study further development of early diagnostic tools using the Apple Watch and iPhone.[1]
Cognetivity Neurosciences (CSE: CGN | OTCQB: CGNSFCSE: CGN | OTCQB: CGNSF) anticipates quick adaptation of its technology on these platforms as well. The company has also announced that it is moving forward with development for launch on Android devices.
Everything promised by these new pharmaceuticals hinges on the success of early diagnosis of mild cognitive impairment, the precursor to the more serious onset of Alzheimer’s and other advances dementias.
Fundamental to both pharmaceutical approaches is a simple, readily available diagnostic tool that detects onset of the disease in the earliest treatable stages.
That’s why Cognetivity Neurosciences stands to rocket into worldwide prominence.
Cognetivity Neurosciences (CSE: CGN | OTCQB: CGNSFCSE: CGN | OTCQB: CGNSF) is a first-of-its-kind artificial intelligence (AI) for early detection of Alzheimer’s and dementia symptoms!
-
Massive investment potential in a desperately needed new medical technology!
-
Early detection promises to revolutionize therapeutic interventions.
-
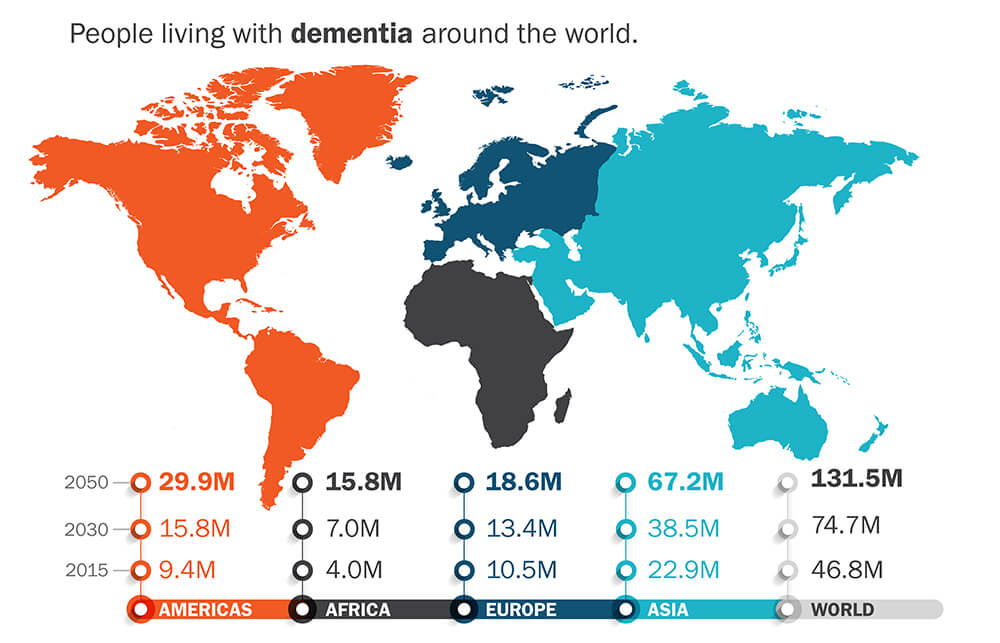
Stunning growth potential with three million new cases added annually in the U.S. alone.
Early detection and diagnosis of Alzheimer’s onset promises to radically improve the quality of life and prognosis for all who are afflicted by this all-to-common, debilitating disease. Cognetivity Neurosciences (CSE: CGN | OTCQB: CGNSFCSE: CGN | OTCQB: CGNSF) groundbreaking technology promises hope for tens of millions worldwide!
This could be the breakthrough that changes everything the world knows for diagnosing and treating Alzheimer’s and dementia patients.
The human cost is horrific…and the financial impact staggering.
Estimates are that dementia already delivers a $1 trillion impact on health care costs and is rocketing toward $2 trillion by end of decade.
The value in the first-ever technology that detects the onset of Alzheimer’s could be worth billions!
At the onset of the disease process, early diagnosis leads to effective interventions that can enhance a patient’s lifestlye. It also allows for more efficient management in advanced stages. Such early detection not only improves patients prognoses, it is key to the enormous cost savings that early diagnosis can afford.
Lastly, and most importantly, as disease modifying therapeutics (now in development) become available, early diagnosis can play a key role in the timing and design of drug regimens.
With FDA approval anticipated within the year, Cognetivity Neurosciences (CSE: CGN | OTCQB: CGNSFCSE: CGN | OTCQB: CGNSF) is a potential home run for early investors
This is a sector already rich with opportunity. For growth investing, investors have scored huge gains on breakthrough medical device technologies. Just last year the sector soared eleven times higher than the S&P.
But this breakthrough in dementia diagnostics could set even greater records!
Cognetivity Neurosciences (CSE: CGN | OTCQB: CGNSFCSE: CGN | OTCQB: CGNSF) stands out with groundbreaking technology reported to diagnose the early onset of Alzheimer’s and related dementias. It promises to open doors to therapies that slow or even stop disease progress well in advance of the time that dementia sets in!

This much-needed technology has been deemed so important that Forbes named Cognetivity as one of “Five Young Companies Making An Impact On The World” in 2019.
Now, Cognetivitiy’s rollout and worldwide distribution could be just months away. As you will read later in this report, the company has already passed a number of key milestones in its progress toward product launch. With FDA approval expected this year, this launch could rocket!
Clearly, this presents ideal timing to get in front of news. Aggressive, growth-focused investors in medical technology stocks could leap on Cognetivity once its progress to market is more widely known.
Start your dues diligence now. You can begin by downloading the company’s latest investor kit from Cognetivity Neurosciences.
Also, contact your broker to put Cognetivity on your investor’s watch list. Stay current with company information and releases. You might also consider making an initial Cognetivity investment to get in on the ground floor. Whatever you decide, now is an ideal time to get started while shares trade ahead of any potential breakout news.
And you can expect a lot of news to be forthcoming!
Two years ago, the company’s much-anticipated Alzheimer’s screening technology achieved two major milestones, which set the pace for rapid advancement to FDA approval anticipated for 2021.
- Cognetivity Neurosciences Presents Groundbreaking New Research Data at the Alzheimer’s Society Annual Conference.May 21st, 2019, Cognetivity presented its groundbreaking new research data after being hand-picked to speak at the Alzheimer’s Society Annual conference 2019 in London.
 Specifically, studies of Cognetivity’s technology showed further proof that diagnosing a delay in the brain’s ability to process visual information, as Cognetivity’s technology is designed to do, positively correlates with the early onset of Alzheimer’s disease. Cognetivity’s (CSE: CGN | OTCQB: CGNSF) technology is thus able to determine subtle changes long before the onset of more severe cognitive symptoms that until now have been the only way to diagnose Alzheimer’s.
Specifically, studies of Cognetivity’s technology showed further proof that diagnosing a delay in the brain’s ability to process visual information, as Cognetivity’s technology is designed to do, positively correlates with the early onset of Alzheimer’s disease. Cognetivity’s (CSE: CGN | OTCQB: CGNSF) technology is thus able to determine subtle changes long before the onset of more severe cognitive symptoms that until now have been the only way to diagnose Alzheimer’s. - A study completed in Q4, 2019 validated the effectiveness of Cognetivity’s key technology in diagnosing Alzheimer’s. Results were published in the influential journal, Nature Scientific Reports. The rigorous standards of this prestigious medical journal mean that the science presented within its pages is of the highest scientific importance.
And progress has been ongoing!
- At the start of 2020, Cognetivity’s Integrated Cognitive Assessment (ICA) technology was awarded regulatory approval by UK’s Medicines and Healthcare products Regulatory Agency. This regulatory approval elevated ICA as a CE-marked medical device, a key milestone on the product deployment timeline. Cognetivity is now authorized for sale and clinical deployment of ICA across the UK and Europe. A few months later, Cognetivity published compelling new data on ICA’s capabilities in the peer-reviewed May 2020 journal of BMC Neurology.
- In August, further evidence of ICA capabilities was presented at the world-leading Alzheimer’s Association International Conference 2020. Through this conference, 31,000+ global attendees were provided exposure to the ICA technology breakthrough.
- By September, Cognetivity had signed its first agreement for distributing ICA technology through the UK National Health Service. In November, the first deployment of that technology released to the port city of Sunderland, England, providing this first-of-its-kind dementia diagnostic tool to general practitioners.
- Finally, in December, the company announced its upcoming research collaboration with Oxford University to pursue expansion of ICA technology onto smartphone-based platforms. This can open pathways to billions of smartphones worldwide and open channels for remote diagnostic evaluation of patients presenting symptoms of cognitive impairment.
These critical 2020 company developments may quickly provide a springboard for future deployments throughout the UK and Europe, then ultimately to the United States and worldwide.
And that may already be underway.
Cognetivity made the final cut in the Texas Medical Center’s Fall 2020 Innovation program. Further, Cognetivity has now been featured in the The Times, The Globe and Mail, and the Wall Street Journal alongside companies such as Microsoft and Apple for the use of artificial intelligence (AI) in early dementia detection.9
This year, 2021, appears to be well on track for international launch of the ICA technology.
In Cognetivity’s current, recently published Investor Information Kit (available for download here) the company announces these key milestones targets year 2021:
- FDA filing and approval
- US commercial launch
- Completion of Android app development
- Corporate Partnerships in Research and Commercialization



These developments could trigger a landslide of industry partnerships for Cognetivity’s AI technology. At the forefront are Biogen, Eisai, and Eli Lilly who are advancing phase 3 trials in dementia therapies. It only makes sense that early diagnosis can be essential to each company’s product rollout. Cognetivity may prove to be essential for their marketing plans.
The value of such above listed developments is immeasurable. With FDA approval anticipated for later this year, Cognetivity Neurosciences (CSE: CGN | OTCQB: CGNSFCSE: CGN | OTCQB: CGNSF) could see its market valuation soar to match or exceed top performers in the medical device sector.
Here are a few examples of the growth potential you’ll find in medical technology stocks!
- Cardiovascular Systems Inc. soared 84% in 12 months on its breakthroughs in treating coronary and peripheral artery disease.
- BioTelemetry Inc. leaped 104% in 12 months on its technology enabling remote monitoring of ambulatory patients’ vital signs.
- Dexcom grew over 640% in the last three years from its innovations in glucose monitoring technology.
- Here’s a real stunner! From its low at the start of 2018, Tandem Diabetes Care rocketed to 2,400% growth on its progress in insulin infusion therapy! 1
Breakthroughs of this magnitude can be expected whenever a medical device addresses significant, persistent patient needs.
For example, Cognetivity’s new technology could revolutionize Alzheimer’s diagnosis in the same manner that digital blood pressure monitors revolutionized heart disease diagnosis
To put that in perspective, since first introduced, the market for digital blood pressure monitors has skyrocketed…and continues to fly.
Fortune Business Insights reports that: “The global digital blood pressure monitor market size stood at USD 666.6 million in 2017 and is projected to reach USD 1,440.3 million by 2025”. 2
Other analysts are even more aggressive in their projections. iHealthcare Analyst forecasts growth to $4 billion by 2023 as digital blood pressure monitors become a routine part of medical exams.
Cognetivity’s Integrated Cognitive Assessment (ICA) technology could be a massive breakthrough for a disease that affects 40 to 50 million people worldwide, and for which there is currently no effective early diagnosis.
With a global population aging fast, the first-ever device for early onset dementia testing could be worth billions.
In fact, research firm Market Research Future forecasts that the Alzheimer’s Disease diagnostic market will reach $12 billion by 2022, six times larger than the blood pressure monitoring device market.
The global health care market stands wide open for Cognetivity’s proprietary, patented technology
It’s a market vacuum with enormous potential. Cognetivity’s first-ever technology is capable of diagnosing Alzheimer’s onset as much as 10-15 years earlier in the disease process.
Early detection is key to successful interventions that prolong quality of life.
Once memory loss has set in, it’s too late to do anything about Alzheimer’s.
“When it’s progressed to that stage, there’s very little that can be done to manage it,” says David Kaufman, PhD, professor of clinical neuropsychology at Saint Louis University. “If we catch it early, we might be able to learn more about what interventions could help and what changes could undo and reverse some of that damage.”
The memory loss that characterizes Alzheimer’s is the result of massive neuronal death, the irreversible loss of brain cells.
“When you’re at the stage of severe memory loss, you have had fairly catastrophic cell death in your brain, and that is not coming back,” says Dr. Thomas Sawyer, Cognetivity’s Chief Operating Officer.
“This is why we confidently call this a groundbreaking medical technology. It will be the first of its kind anywhere in the world and given its relatively low cost and ease of deployment, should achieve rapid acceptance among all medical communities.”
No easily available diagnostic test can detect Alzheimer’s in its early stages. And that is the single biggest factor that makes Cognetivity’s ICA test the most exciting development in Alzheimer’s research today.
Forbes names Cognetivity as one of “Five Young Companies Making an Impact on the World”.
Forbes recently featured Cognetivity Neurosciences (CSE: CGN | OTCQB: CGNSFCSE: CGN | OTCQB: CGNSF) as one of just five companies leading the market with pioneering medical technology, writing that “By diagnosing patients with dementia earlier, doctors will be able to provide faster treatment and ongoing monitoring that can radically improve patients’ lives.”
Forbes’ recognition of Cognetivity’s achievements help validate the revolutionary nature of the technology and the tremendous impact it could have worldwide. The World Alzheimer Report, which includes academics at King’s College London and the Karolinska Institute in Stockholm, confirmed the medical need as “the most significant health and social crisis of the 21st century.”

As a prospective investor you’ll also be pleased to learn that Cognetivity expects to generate revenue even before FDA and EU approval.
In November, 2018, Cognetivity announced a pivotal commercial agreement with global digital healthcare provider dacadoo, a Zurich-based technology company that combines mobile technologies, social networking, Artificial Intelligence (AI) and big data analytics with the aim of helping users improve their health and wellbeing.
Landing dacadoo was a major step forward as the company was recently named one of Europe’s top digital pioneers by the Financial Times.
Cognetivity’s (CSE: CGN | OTCQB: CGNSFCSE: CGN | OTCQB: CGNSF) ICA home testing device will be made available to dacadoo’s 10M+ users through its corporate partners in health and life insurance.8
Revenue is projected from an ongoing, per-user, subscription basis. This could be a huge start for company growth.
According to a report by Transparency Market Research, the global digital health market that Cognetivity enters was valued at $180 billion in 2016. But that has leaped dramatically.
Now, thanks in large part to the rapid penetration of digital communications technology, the digital health market is projected to soar around two-and-a-half-fold to reach roughly $535 billion by 2026!
Take the first step that puts you on board...
With such massive market potential and a truly breakthrough medical technology, Cognetivity is a company for you act on quickly – before the window of opportunity slams shut!
Here’s another way to look at this. Alzheimer’s is the most common type of dementia and the 6th leading cause of death in the United States. And the cost is staggering. According to the Alzheimer’s Association, early intervention could collectively save $7 trillion to $7.9 trillion in long-term care costs.
In America, one new patient is developing Alzheimer’s disease every 65 seconds – resulting in nearly half a million new cases this year alone – demand for an early and accurate diagnostic test cannot be overstated.
Dr Carol Routledge, Director of Research at Alzheimer’s Research UK, made this key observation about Cognetivity’s testing platform. This test is so simple and easily deployed “…you can roll this out on a population-wide basis.”

Rollout can scale to market with incredible speed.
Cognetivity’s patented technology is a software-based test that can reside on a simple handheld device. For launch, the technology will operate on Apple’s iPad device. On that platform, worldwide deployment can scale with remarkable speed. The hardware is already in place!
Apple recently announced that it sold over 500 million iPads during the last ten years and that number is growing by around 40 million annually!
Given that rollout potential and the prospect for near-term approval from the FDA…investors considering a ground floor, early-entry position are encouraged to begin their due diligence into Cognetivity Neurosciences (CSE: CGN | OTCQB: CGNSFCSE: CGN | OTCQB: CGNSF) without delay.
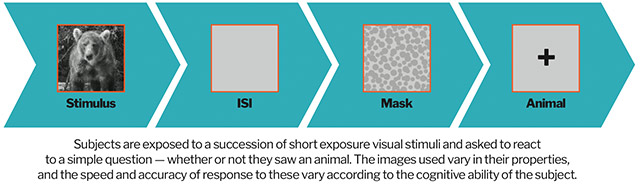
How it works:
Cognetivity holds significant potential to disrupt the world’s most pressing healthcare crisis –making Cognetivity Neurosciences an immediate consideration for a wealth-building investor.
And it’s not just effective in diagnosing Alzheimer’s.
Cognetivity’s technology is also reported to be an effective tool for early diagnosis of a wide range of neurological disorders. These include Parkinson’s disease, sports-related concussions, ADHD, and Multiple Sclerosis.
Now is the time to act. This year could be the breakout year for Cognetivity Neurosciences (CSE: CGN | OTCQB: CGNSFCSE: CGN | OTCQB: CGNSF) . You do not want to be caught on the sidelines!
6 Reasons Investors Should Consider Adding Shares of Cognetivity Neurosciences Today
- Massive Potential Market — According to the World Economic Forum, dementia is the biggest healthcare challenge of the 21st century…a crisis with a staggering $1 trillion-per-year impact on the global economy.
- Game-Changing Early Diagnosis Potential — Cognetivity is aggressively working to tackle the global dementia crisis by bringing a potentially game-changing testing platform to market. Cognetivity’s high-tech solution will help enable early detection of dementia – a breakthrough that is badly needed – and one that can open a door to appropriate future care and treatment.
- Superior A.I. Tech Testing Platform — Cognetivity has developed a testing platform that looks to be superior to – and has a number of critical advantages over – existing testing methods. This software-driven solution could allow cognitive testing to be as simple, inexpensive and prevalent as an annual blood pressure check.
- First to Market…ZERO Competition! The market for cognitive testing is massive – yet no other company has developed a realistic solution. Thanks to a lack of competitors and its comprehensive patent protection, Cognetivity should have a significant first- to-market advantage over any potential competitors.
- Hardware Platform Already in Place — Cognetivity’s software launches on Apple’s iPad device. Apple reports having sold over a half billion iPads worldwide; a number that continues to grow at around 40 million devices per year.
- Early Alzheimer’s Diagnoses Will be Key to Future Drug Utilization — Currently, over 100 advanced clinical trials are being conducted for Alzheimer’s pharmaceuticals. With many new drugs anticipating FDA approval, Cognetivity’s early diagnostic AI could play an essential role in each drug manufacturer’s marketing programs.
Get Started Now:
Here’s where to immediately download the recently updated Cognetivity Investor Kit and to register your contact information for future updates and news releases!
Learn More About Cognetivity Neurosciences (CSE: CGN | OTCQB: CGNSFCSE: CGN | OTCQB: CGNSF) at your brokerage today!
1 https://www.fool.com/quote/nasdaq/tandem-diabetes-care/tndm/
2 https://www.fortunebusinessinsights.com/industry-reports/digital-blood-pressure-monitors-market-100066
3 https://www.msn.com/en-us/news/technology/apple-has-sold-more-than-500-million-ipads-over-the-last-decade/ar-BB194oO7
4 https://www.alz.org/news/2018/new_alzheimer_s_association_report_reveals_sharp_i
5 https://www.weforum.org/agenda/2017/09/dementia-trillion-dollar-global-crisis/
6 http://www.health.gov.au/internet/ministers/publishing.nsf/Content/health-mediarel-yr2016- wyatt011.htm
7https://www.forbes.com/sites/alisoncoleman/2018/12/30/five-young-companies-making-an-impact-on-the-world-to-watch-in-2019/?sh=51789b01ac47
8https://www.startupticker.ch/en/news/may-2019/dacadoo-sees-strong-revenue-growth
IMPORTANT NOTICE AND DISCLAIMER
This website is owned and hosted by Market Tactic Media Ltd. Articles appearing on this website should be considered paid advertisements. Market Tactic Media Ltd. and its owners, managers, employees, and assigns (collectively “the Publisher”) is often paid by marketing companies to host websites on which articles profiling public companies are published. The Publisher has not been compensated by any of the profiled companies. The Publisher’s compensation for articles appearing on this website is as follows:
- The Publisher has been paid approximately $500 per week while the advertisement campaign was active by Think Ink Marketing as compensation to host the article profiling Cognetivity Neurosciences.
The Publisher has not participated in the creation of the content of any articles appearing on this website and so cannot guarantee the accuracy or completeness of the information in any of the articles. The Publisher expressly disclaims any responsibility or liability for statements made in any of the articles.
SHARE OWNERSHIP. The Publisher does not own any shares of any profiled company and has no information concerning share ownership by others of any profiled company. The Publisher cautions readers to beware that third parties, profiled companies, and/or their affiliates may liquidate shares of the profiled companies at any time, including at or near the time you read the articles on this website and this has the potential to hurt share prices. Frequently companies profiled in such articles experience a large increase in volume and share price during the course of investor awareness marketing, which often ends as soon as the investor awareness marketing ceases.
NO SECURITIES OFFERED. The articles on this website are not, and should not be construed to be, offers to sell or solicitations of an offer to buy any security. Neither the articles on this website nor the Publisher purport to provide a complete analysis of any company or its financial position. The Publisher is not, and does not purport to be, a broker-dealer or registered investment adviser. The articles on this website are not, and should not be construed to be, personalized investment advice directed to or appropriate for any particular investor. Any investment should be made only after consulting a professional investment advisor and only after reviewing the financial statements and other pertinent corporate information about the company. Further, readers are advised to read and carefully consider the Risk Factors identified and discussed in the profiled company’s SEC and/or other government filings. Investing in securities, particularly microcap securities, is speculative and carries a high degree of risk.
INDEMNIFICATION/RELEASE OF LIABILITY. By reading articles on this website, you acknowledge that you have read and understood this disclaimer, and further that to the greatest extent permitted under law, you release the Publisher, its affiliates, assigns and successors from any and all liability, damages, and injury from articles appearing on this website. You further warrant that you are solely responsible for any financial outcome that may come from your investment decisions.
LINKS TO THIRD PARTY WEBSITES. This website enables users to link to external websites not under the control of The Publisher. The Publisher has no control over the nature, content, and availability of those sites. The inclusion of any links is not intended as, and should not be construed as, a recommendation or endorsement of the content or views expressed on such external websites. The Publisher expressly disclaims any representation concerning the quality, safety, suitability, or reliability of any external websites and the content and materials contained in them. It is important for users to take necessary precautions, especially to ensure appropriate safety.
INTELLECTUAL PROPERTY. The Market Tactic is the Publisher’s trademark. All other trademarks used in this communication are the property of their respective trademark holders. The Publisher is not affiliated, connected, or associated with, and is not sponsored, approved, or originated by, the trademark holders unless otherwise stated. No claim is made by the Publisher to any rights in any third-party trademarks.Thin
Read full disclaimer here.